Roma 10 maggio 2023
La Biotech Takis in prima linea per lo sviluppo di soluzioni contro patogeni ad alto potenziale epidemico/pandemico.
La Biotech Takis in prima linea per lo sviluppo di soluzioni contro patogeni ad alto potenziale epidemico/pandemico.

“Siamo entusiasti per la recente acquisizione dell’innovativo modulo BSL-3, uno strumento di ultima generazione per isolare e manipolare in condizioni di massima sicurezza patogeni pericolosi per l’uomo, come SARS-CoV-2. Questa nuova struttura rappresenta per Takis, per il territorio e per il Paese l’opportunità di raccogliere una sfida più che mai attuale nel nostro tempo: abbracciare un nuovo approccio alla ricerca contro le malattie infettive che tenga conto del rischio di future pandemie.” dichiara Luigi Aurisicchio, CEO/CSO della Takis.
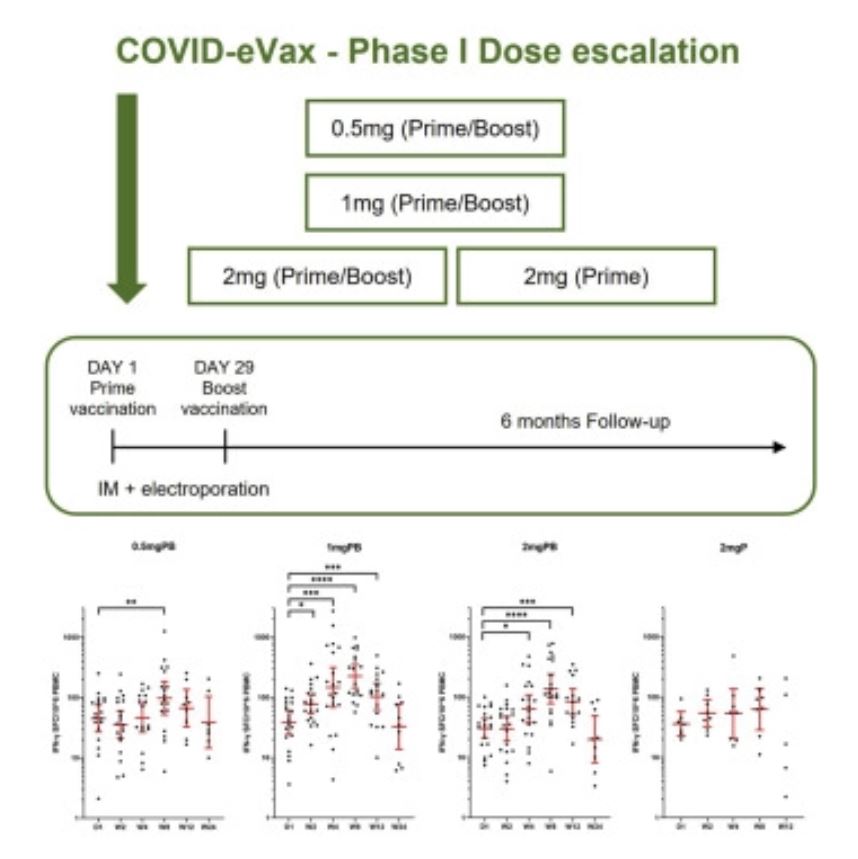
“Dopo aver realizzato un vaccino contro Covid-19 che ha raggiunto con successo la Fase I della sperimentazione clinica, i nostri ricercatori avranno adesso la possibilità di svolgere ricerche sempre più avanzate che aiuteranno a prevenire e ad affrontare possibili pericoli futuri“ continua Emanuele Marra, Chief Operating Officer alla Takis. “Il vaccino COVID-eVax sviluppato alla Takis ha infatti seguito un percorso di sperimentazione che nasce da Castel Romano già a gennaio 2020 ma solo avvalendosi di collaborazioni nazionali ed internazionali, ha potuto dimostrare la sua efficacia contro SARS-CoV-2 in ambiente BSL-3” continua Emanuele Marra, COO della Takis.
“Tramite questo modulo innovativo potremo dunque valutare velocemente e in tutta sicurezza nuove molecole, vaccini o anticorpi monoclonali per il trattamento o la prevenzione di malattie infettive ad alto rischio di diffusione. Ad esempio per SARS-CoV-2 siamo riusciti a sviluppare in pochissimo tempo vaccini specifici e una batteria di potenti anticorpi contro le varianti emergenti del virus. Non solo. In collaborazione con l’Accademia, la Takis è molto attiva sul territorio Laziale per la realizzazione di laboratori condivisi e per la formazione di giovani ricercatori ad elevata specializzazione, di cui oggi l’Italia è ancora carente rispetto ad altri paesi” conclude Giuseppe Roscilli, CTO della Takis.
La BSL-3 rappresenta quindi un importante investimento sulla ricerca italiana e sulla tutela della salute di tutti noi.